Geometric Isomerism :
`=>` This type of isomerism arises in heteroleptic complexes due to different possible geometric arrangements of the ligands.
`=>` Important examples of this behaviour are found with coordination numbers `4` and `6`.
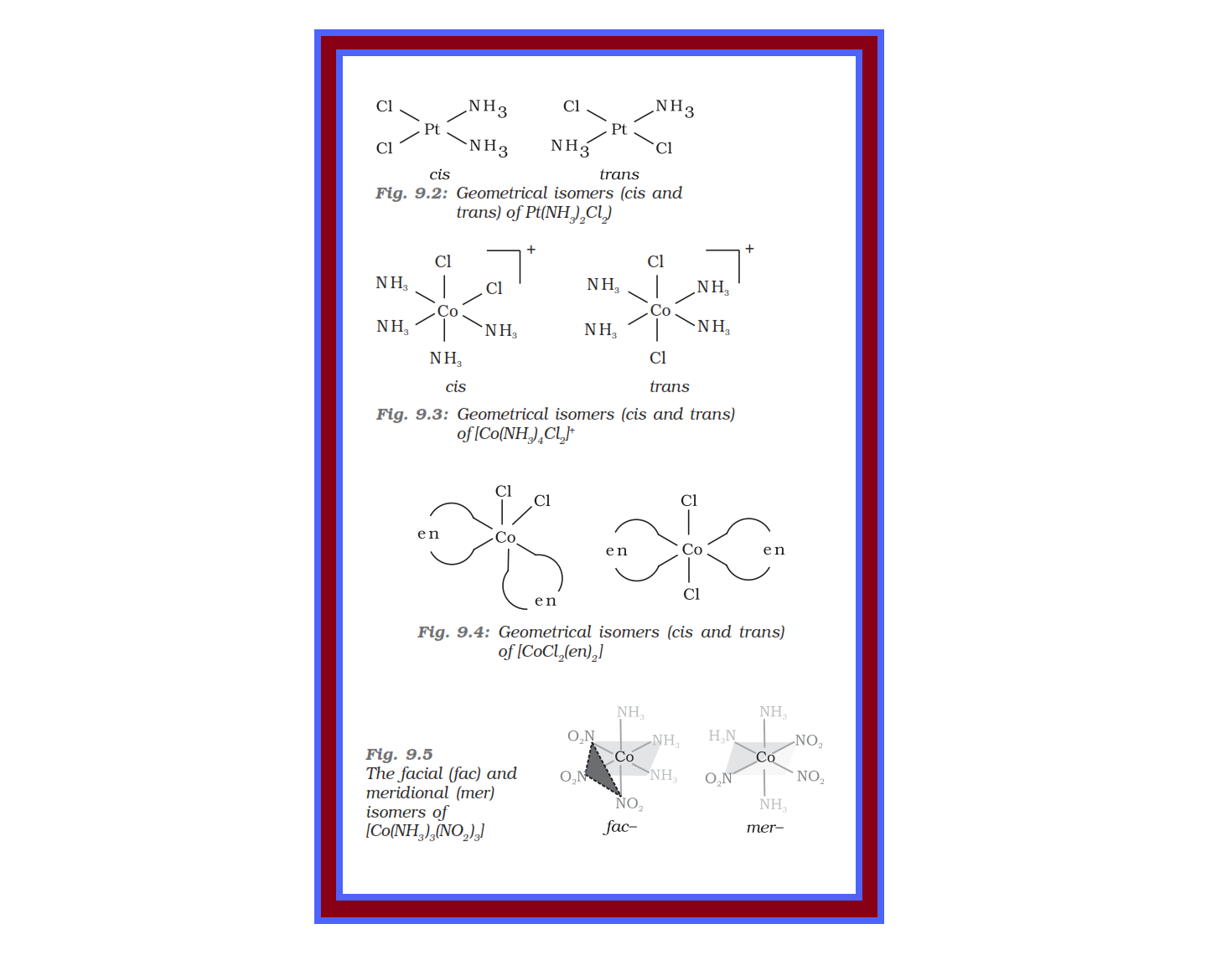
`=>` In a square planar complex of formula `color{red}([MX_2L_2])` (`color{red}(X)` and `color{red}(L)` are unidentate), the two ligands `color{red}(X)` may be arranged adjacent to each other in a c is isomer, or opposite to each other in a trans isomer as depicted in Fig. 9.2.
`=>` Other square planar complex of the type `color{red}(MABXL)` (where `color{red}(A, B, X, L)` are unidentates) shows three isomers-two cis and one trans.
`=>` Such isomerism is not possible for a tetrahedral geometry but similar behaviour is possible in octahedral complexes of formula `color{red}([MX_2L_4])` in which the two ligands `X` may be oriented cis or trans to each other (Fig. 9.3).
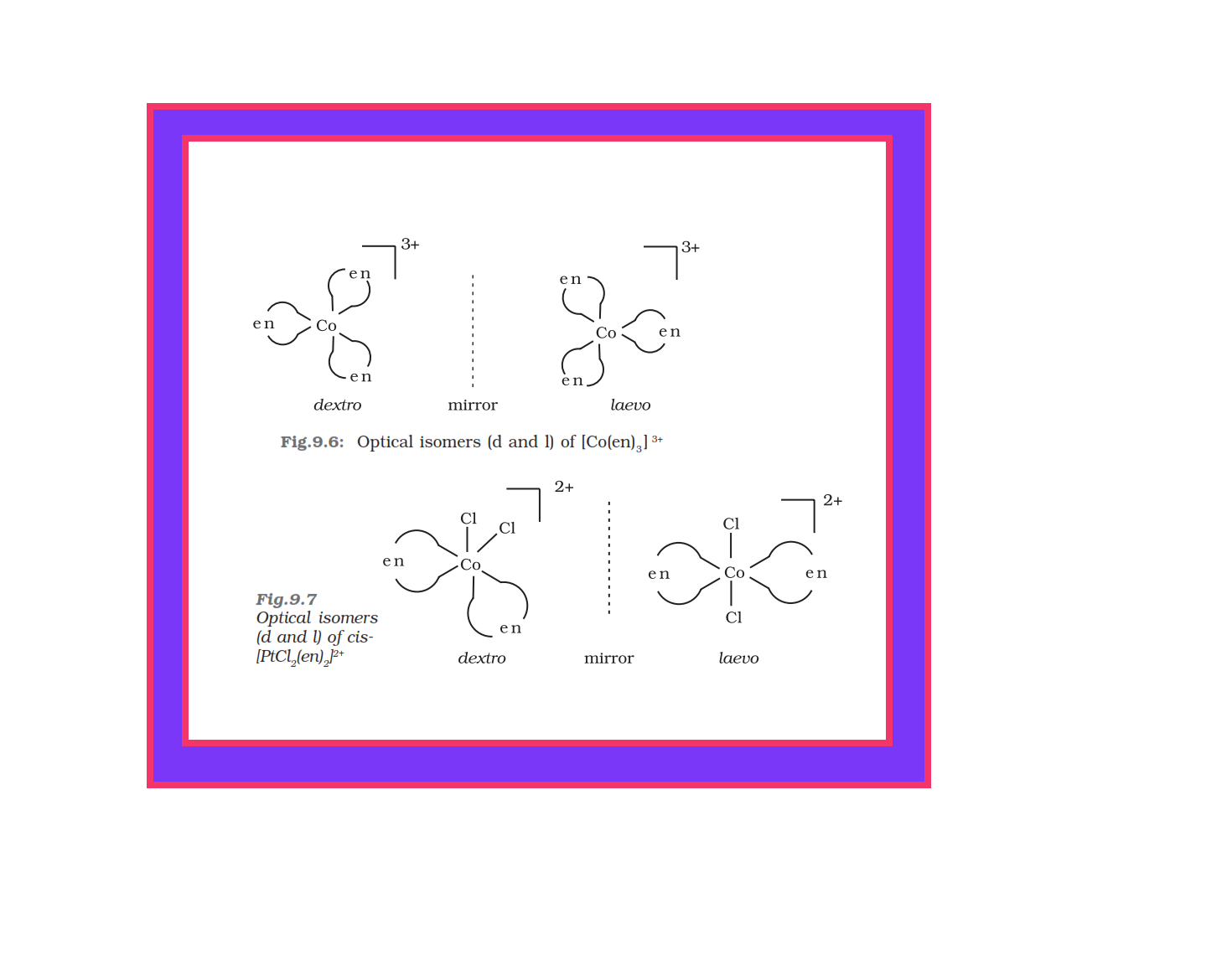
`=>` This type of isomerism also arises when didentate ligands `color{red}(L – L)` [e.g., `color{red}(NH_2 CH_2 CH_2 NH_2 (en))`] are present in complexes of formula `color{red}([MX_2(L – L)_2])` (Fig. 9.4).
`=>` Another type of geometrical isomerism occurs in octahedral coordination entities of the type `color{red}([Ma_3b_3])` like `color{red}([Co(NH_3)_3(NO_2)_3])`.
`=>` If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac)
isomer. When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer (Fig. 9.5).
`=>` Important examples of this behaviour are found with coordination numbers `4` and `6`.
`=>` In a square planar complex of formula `color{red}([MX_2L_2])` (`color{red}(X)` and `color{red}(L)` are unidentate), the two ligands `color{red}(X)` may be arranged adjacent to each other in a c is isomer, or opposite to each other in a trans isomer as depicted in Fig. 9.2.
`=>` Other square planar complex of the type `color{red}(MABXL)` (where `color{red}(A, B, X, L)` are unidentates) shows three isomers-two cis and one trans.
`=>` Such isomerism is not possible for a tetrahedral geometry but similar behaviour is possible in octahedral complexes of formula `color{red}([MX_2L_4])` in which the two ligands `X` may be oriented cis or trans to each other (Fig. 9.3).
`=>` This type of isomerism also arises when didentate ligands `color{red}(L – L)` [e.g., `color{red}(NH_2 CH_2 CH_2 NH_2 (en))`] are present in complexes of formula `color{red}([MX_2(L – L)_2])` (Fig. 9.4).
`=>` Another type of geometrical isomerism occurs in octahedral coordination entities of the type `color{red}([Ma_3b_3])` like `color{red}([Co(NH_3)_3(NO_2)_3])`.
`=>` If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac)
isomer. When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer (Fig. 9.5).